 ALERT
ALERT **Important for iLab Users**
The Help link in iLab opens an email to contact Support. To submit or track requests, visit: Submit and Track Support Requests
The Program in Cellular and Molecular Medicine (PCMM) at Children's Hospital operates a Microscopy Core with advanced instrumentation in Light and Electron Microscopy. While Electron Microscopy can provide the ultimate in resolution, modern Light Microscopy such as Confocal and Multiphoton has the advantage of imaging biochemical process in real time, shedding light on the vastly complex molecular world of cells and tissues. We operate a dedicated Multiphoton-Intravital Microscopy (MP-IVM) Facility, since the year 2000, on the second floor of the Warren Alpert Building at the Harvard Quad. We were the very first to provide MP-IVM service to Longwood area scientists since that time, with expertise training relevant to the experiment being pursued.
All of the microscopes at the PCMM Microscopy Core are shared equipment and are available to both internal and outside users. However, PCMM users are given priority.
(a) Read Me First (b) About training materials and videos
Please note that training materials posted here may not have the most up-to-date protocols, always check with the facility manager for any updated information.
System 1 -Confocal-
Olympus Fluoview FV1000 point scanning confocal system
Configuration: Inverted, with Olympus IX 81 microscope
Lasers lines: 405, 457, 488, 515, 559, 635 nms
Detectors (PMTs): 4 fluorescence +1 transmission; DIC and spectral imaging capability
Location: 3 Blackfan Circle, 3rd/Fl., Rm03123
FV1000 Instructional Videos: (1) Prerequisite Exercise (2) Starting Up (3) Shutting Down (4) Getting the First Image (5) Collecting a Confocal Stack (6) Merging Channels (7) Hi-Lo Adjustment (8) Zooming
Operational Tips and Resources : (1) About Large 24X60mm Coverslips (2) Proper Handling of the Slide Holder

System 2 -Multiphoton-
Olympus Fluoview FV1200 multiphoton system
Configuration: Upright, with Olympus BX 61 WI microscope
Lasers: Spectra Physics MaiTai DSHP, tuning range = 690-1020 nm
Detectors: 4 external (non-descanned)
Location: 200 Longwood Ave., WAB Rm274A
FV1200 MPE Instructional Videos: (1) Starting Up (2) Getting the first image (3) Shutting Down
Operational Tips and Resources: (1) CBR Retreat Poster 2002 (2) IDI Retreat Poster 2009
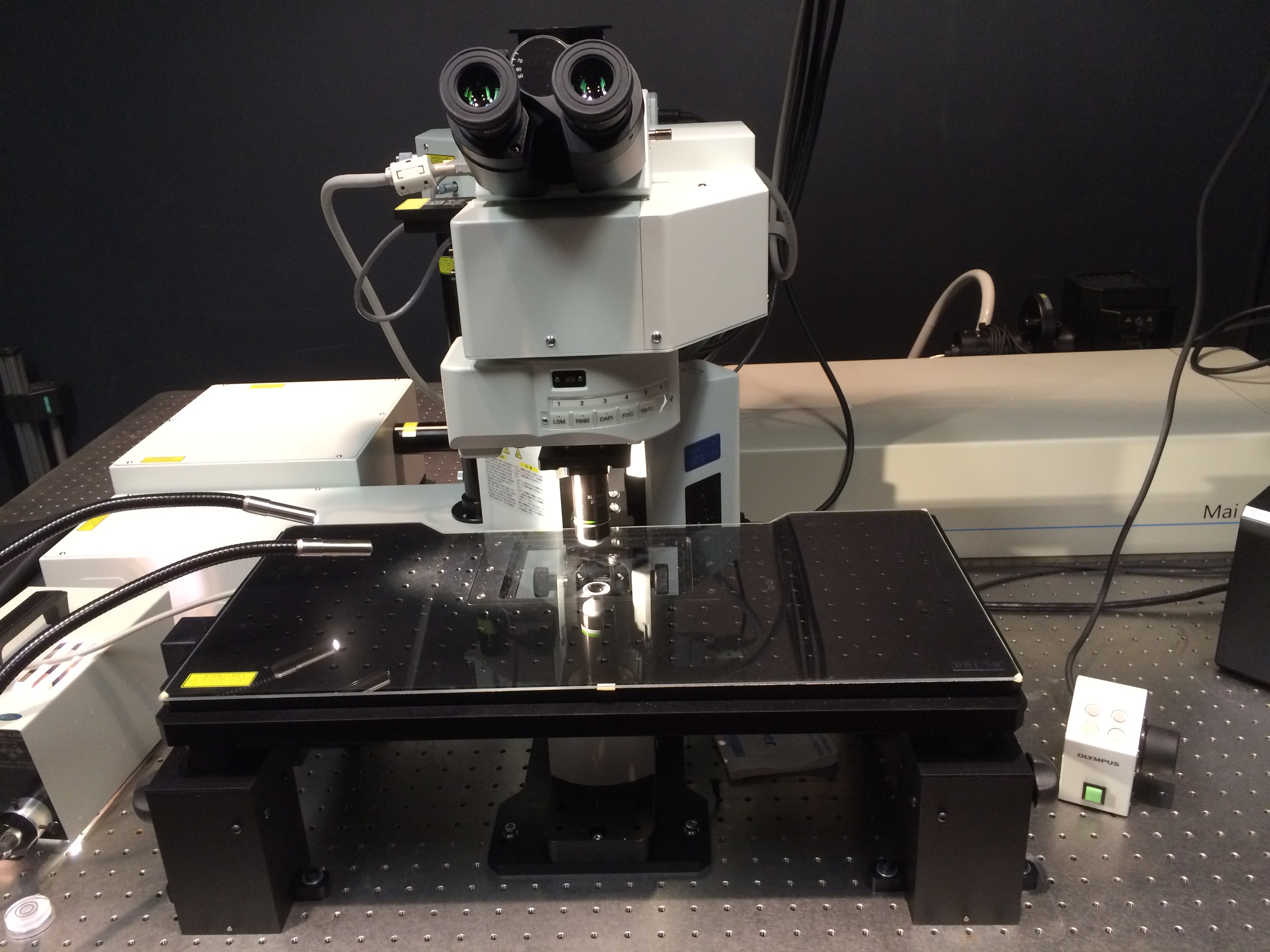
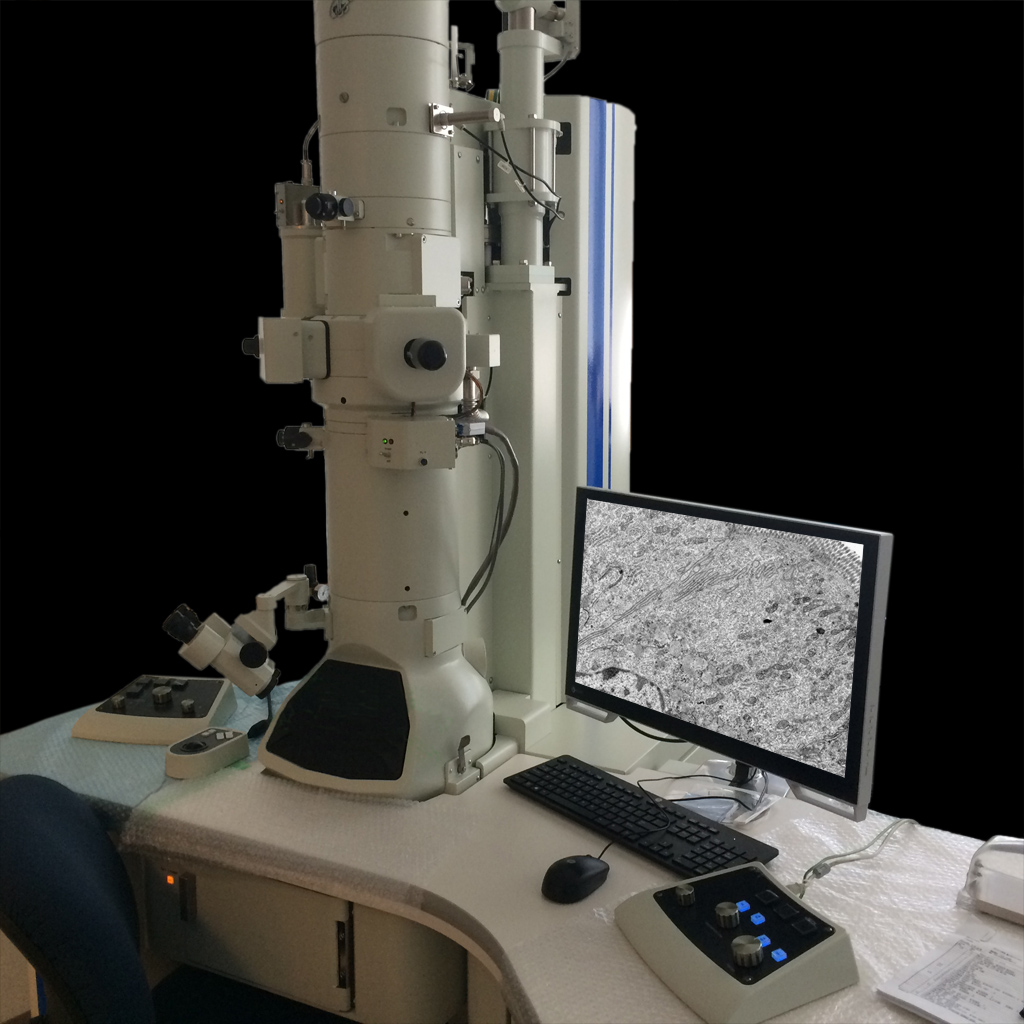
System 4 -TEM-
Joel JEM1400 Transmission Electron Microscope
Features: 80,100 or 120 KV accelerating voltage with LaB6 cathode; tilting cryostage; 16MP cooled CCD camera
Location: 3 Blackfan Circle, 3rd/Fl., Rm03078
JEM 1400 Instructional Videos: (1) Starting Up (2) Changing Specimen (3) Taking and Saving Images (4) Specimen Airlock exposed
Operational Tips and Resources: (1) Advanced Technique - Finding that focus (a) video (b) pdf (2) PCMM Retreat Poster 2018 (3) PCMM Retreat Poster 2019
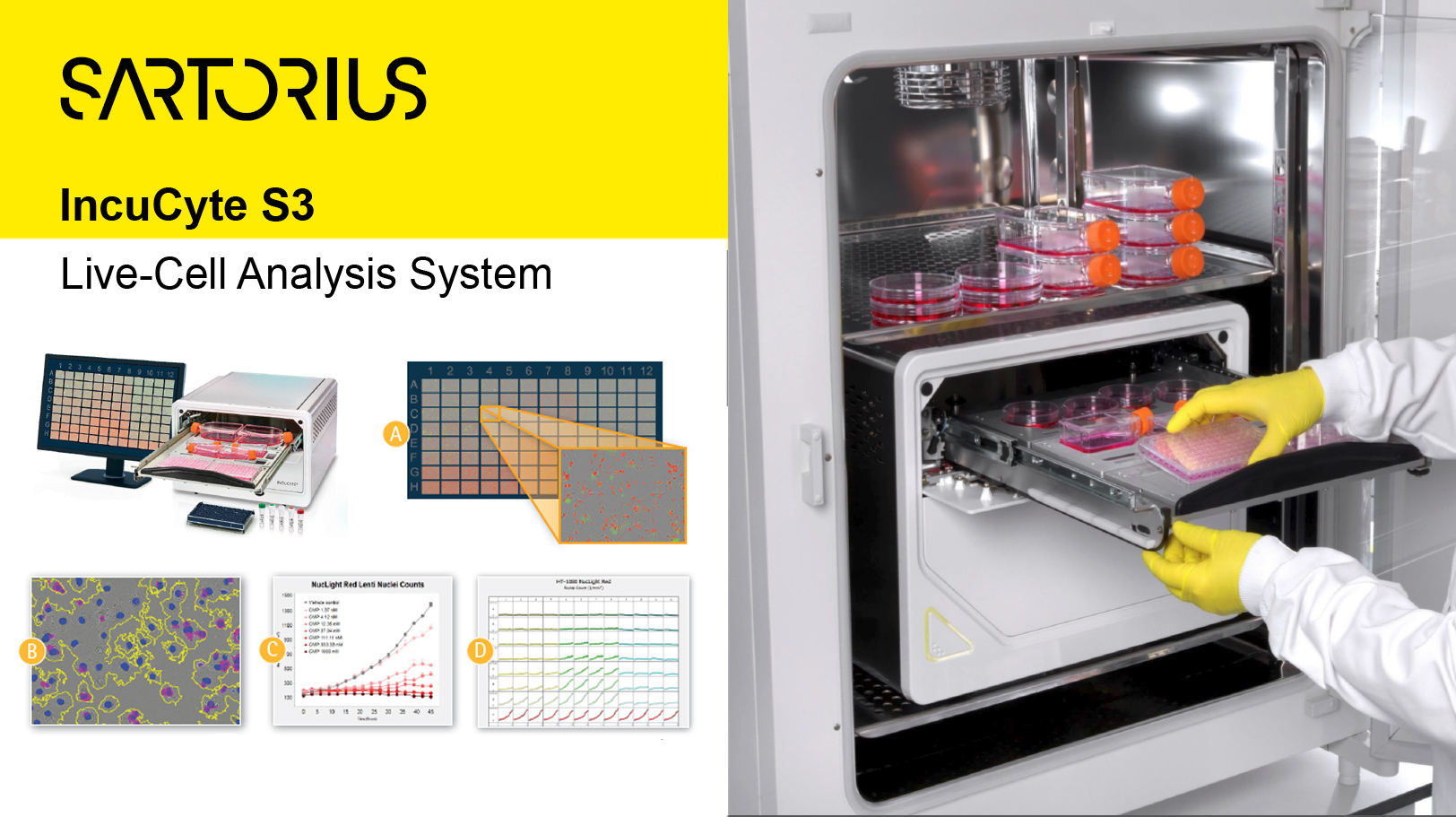
System 5 -Live-Cell Analysis-
Sartorius IncuCyte S3 Live-Cell Analysis System
Capabilities: automated collection, measurement and analysis of culture cells in situ, for short/long term time lapse experiments
Location: 210 Longwood Ave., Amenise 2 Rm235
Harry Leung - Director
Email: harry.leung@childrens.harvard.edu
Phone: 617-713-8299
| Hours | Locations |
|
Training or Assisted Use: 9:00am - 5:00pm (Monday - Friday); Self-Use (for trained users): All Hours (24/7) |
200, 210 Longwood Ave, Boston, MA 02115; 3 Blackfan Circle, 3/F Boston, MA 02115 |
| Name | Role | Phone | Location | |
|---|---|---|---|---|
| Harry Leung |
Director
|
617.713.8299
|
harry.leung@childrens.harvard.edu
|